Every cancer is unique because every person is unique, and one of the most important weapons in any cancer battle is information. Isabl offers that in abundance through rapid sequencing of cancer cells’ entire genomes, potentially showing which therapies will and won’t be effective within days. The company has received a breakthrough designation from the FDA and raised $3 million to bring its approach to market.
The last 10 years have brought numerous medical advances due to the commoditization of genomic processes from sequencing to analysis, and cancer treatment is no exception. In fact, because cancer is (though it is a simplification) a genetic mutation that has gotten out of hand, understanding those genes is an especially promising line of research.
Panel tests look within the DNA of cancerous cells for mutations in a selection of several hundred genes known to affect prognosis and clinical strategy. For instance, a cancer may have certain mutations that render it susceptible to radiation treatment but resistant to chemo, or vice versa — it’s incredibly helpful to know which.
Isabl co-founder and CEO Elli Papaemmanuil explains that however helpful panel tests are, they’re only the beginning.
“These tests have been designed very carefully to look for the most common mutations, and they have revolutionized cancer diagnosis for patients with common cancers,” she said. “But patients with rare cancers — and what we define as a rare cancer is still a third of patients — don’t benefit from them.”
Even many with common cancers may find that their condition does not involve mutations of these most predictive genes. The relevant genes are somewhere among the other two billion base pairs — current tests only look at about 1% of the genome.
While the technology exists to look at that other 99%, it has historically been expensive and slow compared with panels, and analysis of the resulting large body of data was likewise difficult and time consuming. But Isabl’s tests show that it’s definitely worthwhile.
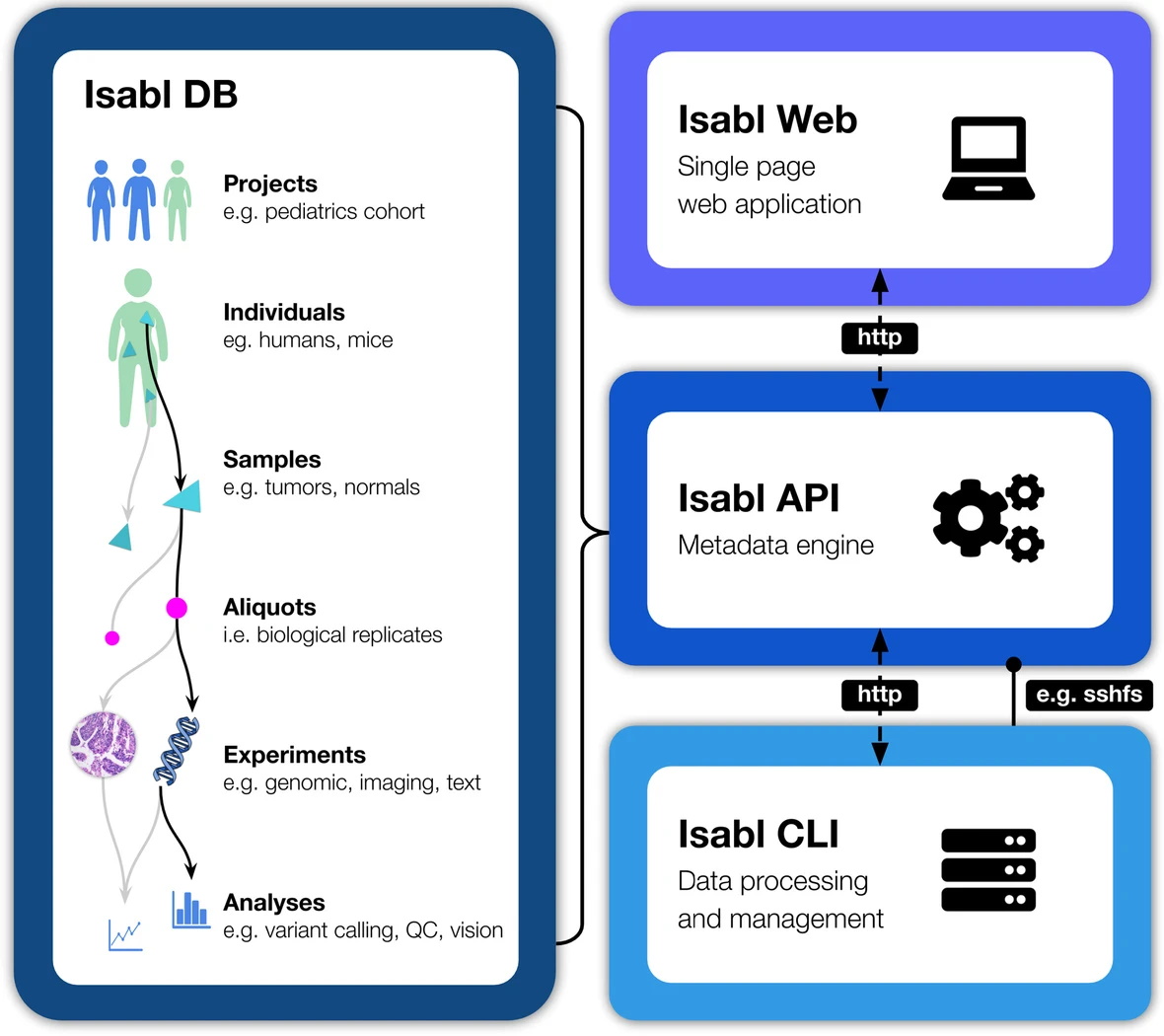
Image Credits: Isabl
“It turns out that whole-genome sequencing can detect many more clinically relevant findings — results we can act on today. And what we’ve done is develop a platform that lets us summarize it in a way that doctors can read and use, in a day,” Papaemmanuil said. They call it a “clinically actionable whole genome and transcriptome test,” or cWGTS.
The company was formed out of research Papaemmanuil did at Memorial Sloan Kettering, a cancer care and science nexus in New York. “You could see all these successes from panel testing, then all these patients who weren’t benefiting. But in my lab we had the tech and the know-how,” she recalled. They collected and combined three different data sets: the germline (i.e., patient’s) genomes; the tumor’s genome and also its transcriptome, essentially what the body produces from transcribing the DNA.
“This gives a really full picture of the profile of the tumor,” she said. “Rather than having a classifier or a model that annotates the mutations [i.e., an automated panel test], we have analytics that integrate those three layers to interpret the role of the mutation and its relevance to each tumor type.”
Though it does own the whole process from sampling to report, Isabl’s key advance is data based and therefore “there is no technical obstacle to making this solution available today. And we’ve demonstrated we can do it at scale,” Papaemmanuil said. But in the medical world, just because it’s possible doesn’t mean it’s permitted. The FDA has granted the technology with “breakthrough” status, which is a fast track — but even the fast track is slow in the federal government.
While full clinical approval is probably 3-5 years away, that’s much faster than the 5-10 years estimated by the industry for this type of application. But research, both for validation and other purposes, is ongoing, having just published the main paper proving out the process today in Nature Communications. (Though this study focuses on pediatric and young adults cancers, the technique is not limited to those demographics.)
“The seed round is very much to let us do the roadmap — it’s a good starting point for getting the necessary evidence and approvals,” Papaemmanuil said. “We’re already partnering with cancer centers to do studies, and most importantly, to hear from oncologists on what they need and how they’d like the data.”

From left, Isabl co-founders Andrew Kung, Elli Papaemmanuil and Juan Santiago Medina. Image Credits: Isabl
The $3 million round was led by Two Sigma Ventures, with participation from Y Combinator, Box 1 and other firms. Papaemmanuil’s co-founders are CTO Juan Santiago Medina and Andrew Kung.
She also made it clear that Isabl’s research would be conducted openly — “We have a very strong scientific foundation and will be active in publishing the work. The data needs to be both published and made accessible in a form that will enable further research,” she said. The self-reinforcing play of producing and identifying predictive data could prove an incredibly valuable resource across many disciplines.
Isabl is an example of the power of a more or less pure data play in an industry more frequently associated with advances in the lab — though of course it took a lot of lab work to produce in the first place. But when automation of key processes, in this case DNA transcription, enables a huge uptick in data capture, there’s always value to be found in it. In this case that value could save many lives.











 English (US) ·
English (US) ·