The appetite for genomic data continues to rise in the field of biotech and pharmaceutical research, but cost is still a factor even sequencing a full genome now costs as little as $1,000. But with claims of reducing that cost by another order of magnitude to $100, Ultima Genomics may even further accelerate this economy.
Ultima says that its sequencing machine and software platform, the UG 100, can perform a complete sequencing of a human genome in about 20 hours, with precision comparable to existing options, but does so at a far lower cost per “gigabase,” which is to say per million base pairs of DNA analyzed.
The technical advances may not be entirely intelligible to people who are not already familiar with how DNA is sequenced, and not being an expert myself I won’t attempt a full explanation. But it helps to understand that essentially the DNA, amplified in a reagent (so basically a lot of the same DNA in a solution), is passed through small channels where fragments bind to certain microscopic mechanisms, which prepare it to be imaged by a lot of base detectors operating in parallel. These sequences are then reassembled into the whole genome by matching their ends together.
Ultima’s claimed advance is threefold. First, rather than having the reagent travel down fluidic channels that must be flushed afterwards in preparation for the next step, the micromachinery (“a dense array of electrostatic landing pads”) is etched onto a 200mm silicon wafer. This well known process uses cheap, readily available stock and can be mass manufactured.
But more importantly, it enables the reagent to be simply deposited in the center of the wafer, which spins to distribute it evenly across its entire surface using centrifugal force. This is efficient, mechanically simple, and allows the resulting sequences to be read “during rotation of the wafer in a continuous process, analogous to reading a compact disc.”
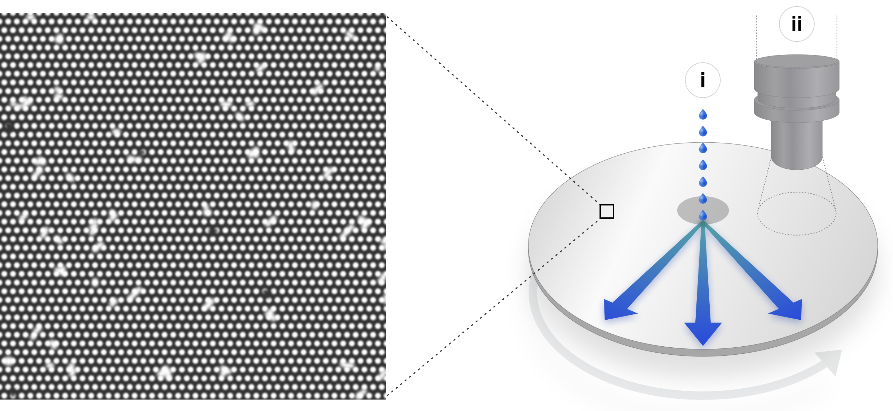
Diagram of the UG 100’s open water process, with an image of the wafer’s micropatterned surface.
The second advance is a little more arcane, having to do with the process of preparing and directly reading the DNA — rather than replacing the bases with more machine-readable ones or relying on dicey particle-level imagery, a clever combination of the two is struck. It’s less destructive to the original strands but also doesn’t require error-prone measurements like individual photon counts.
The third advance involves machine learning to accelerate the process of turning optical data (the CD-style scanning signal) into usable data. A deep convolutional neural network trained on multiple genomes and fragments is tuned based on a sample from the genome being sequenced, then set to work verifying and assembling all the tiny pieces of data into the whole genome. This process speeds things up and eliminates error.
There is considerable margin for improvement on the process, primarily in the size and density of the wafer and its surface, leading to improved throughput. This could push the price lower, but for now a 90% reduction is more than enough to go to market with.
Founder and CEO Gilad Almogy (also the first author of many on the paper quoted above), said that the company is currently working with early access partners to put out some early proof of concept studies showing the capabilities of the sequencing technique. The first of these, collaborations with the Broad Institute, Whitehead Institute, the Baylor College of Medicine and more are being presented soon or currently available as preprints.
Broader commercial deployment is expected in 2023 (final pricing is undetermined but will likely reflect the advantage conferred by this method over others). I asked Almogy what he felt were the areas of the biotech and medical industry that will benefit most from this new capability.
“We believe genomics will be the first line diagnostic across diseases,” he said, pointing out that it is complementary to many existing techniques and only improves understanding of them.
But the far lower cost could lead to genomic population studies, improving our general understanding of systematic variance in the genome across different groups and over time. “We’re already talking with partners who are interested to do more genomes, but also RNA expression and proteomics at a population scale, said Almogy. This is also key to epigenetic studies that look at methylation and other ways our DNA changes as we age.
“Deep oncology,” or using genetic profiling to characterize and fight cancers, may be one of the earliest clinical applications — and in fact Isabl is way ahead of him on that one. The company’s quick turnaround whole-genome tumor sequencing could be made even quicker.
Similarly, single cell sequencing (e.g. a blood cell or neuron) could help in both clinical and research environments, but “the cost of sequencing also prevents us from routinely using single cell sequencing for applications like immune profiling,” Almogy said. Reducing the cost so considerably could change that equation.
With sequencing reduced from a billion-dollar process to one you could get done monthly if you wanted to and have it covered by insurance, the biotech industry seems to be on the precipice of yet another data explosion, beyond the scale of the unprecedented one we are already in the midst of. With companies like Ultima multiplying data volumes, the next opportunity is likely to be not in production but management and utilization of this newly deepened sea of information.











 English (US) ·
English (US) ·